SUPRAMOLECULAR CATALYSIS: RE-PURPOSING THE USE OF METALLOPORPHYRINS
Inspired from Nature, where weak interactions are responsible for the unique efficiency of metalloenzyme’s activity and selectivity, we study the behavior of substrates able to interact via reversible interactions with appropriately designed supramolecular catalysts. As such, new issues reminiscent from natural selection processes that cannot be addressed by traditional approaches will be tackled; for example substrates (or products) inhibition, substrate selectivity, etc . For that, we have pioneered the use of reversible coordination chemistry as a tool for the design of supramolecular catalysts around porphyrins in which the molecular recognition event takes place inside the porphyrin core whereas the reactivity occurs in a peripheral side. As such, fine-tuning of the distance between the active site and the substrate binding site leads to new levels of activity and selectivity for challenging chemical transformations.
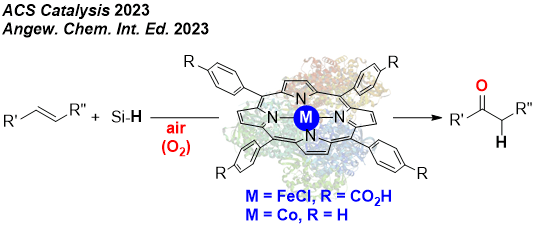
REPLACING NOBLE METALS BY FIRST ROW METAL CATALYSIS
We have initiated a new research line aiming at the replacement of palladium catalysis in oxidation chemistry, namely the Wacker-type oxidation of olefins into ketones. We have disclosed that iron- and cobalt-based porphyrins are suitable catalysts for accessing ketones starting from olefins under ambien pressure and temperature using hydrosilanes as additional reagents. This results follow the trend of Green Chemistry. We also developed iron and cobalt electrocatalysts for the production of dihydrogen from acidic or neutral water conditions.